A pharmaceutical document management system (DMS) is a necessity for pharmaceutical companies across the globe, from the USA and Canada to Australia and Japan. This system helps tackle strict regulations, facilitating role-based access, operational checks, electronic signatures, and time-stamped audit trails. Cloud, ML, and blockchain are among the technologies that improve security and drive automation of DMS. You should also consider implementing document standardization, version control, and records management – a process Exoft can assist with.
FDA conducts regular inspections to ensure compliance in pharmaceutical documentation. The number of recorded deficiencies rises annually. For example, in
2023, the administration noticed the lack of written procedures and issues with computer control of master formula records. Such deficiencies may cost companies billions of dollars. Data protection non-compliance can double the risks. All it takes to prevent them is implementing a reliable document management system for the pharmaceutical industry.
In this post, we’ll share our experience with
healthcare software development services
and documentation management, including a brief overview of the regulatory framework, documentation management best practices, and must-have features.
Overview of International Regulatory Requirements
National governing authorities have varying electronic pharma document management requirements. The jurisdiction your business falls under will dictate compliance requirements. You can find some of the relevant regulations in the table below.
At the same time, basic requirements for pharmaceutical documentation processes are similar worldwide. They stipulate the need for:
- Limited access for authorized personnel only
- Continuous checks of operational systems, authority, and devices
- Electronic signature use regulations
- Computer-generated time-stamped audit trails
- Enabling investigator access during inspections
- Records protection and retention in a format ready for retrieval
Digitizing document management for pharmaceutical companies is further complicated by robust legacy systems that pose additional challenges regarding record digitization, validation, and accessibility.
Leveraging Technology for Document Management
As the volume of records increases, so does the regulatory load. Therefore, implementing electronic document management in pharma becomes essential. Leveraging disruptive technologies, ranging from IoT to blockchain and AI, can help achieve compliance in pharmaceutical document management.
Cloud-Based Systems
Although many organizations embrace the return to office spaces, others are shifting towards hybrid workplaces. As a result, the need for efficient collaboration tools rises. Cloud-based solutions for pharmaceutical document control combine security with accessibility. Cloud operators take over the physical and server-side security aspects, letting pharmaceutical companies focus on user-side security measures, like role-based access and multi-factor authentication. With these in place, your organization can:
- Leverage interstate or international partnerships
- Speed up data sharing within the industry
- Accelerate new medicine development
- Establish centralized monitoring
- Encourage the development of standardized regulatory requirements
Machine Learning and Artificial Intelligence
Medical researchers rely on AI to process large data sets, improve prediction accuracy, and automate routine processes. However, it also helps with a pharmaceutical document management system. For example, ML-powered image processing can help digitize handwritten documentation and make it searchable. Besides, an AI-powered
medical information system
can structure large volumes of legacy data, generate reports, and highlight inconsistencies in version history.
Blockchain
Blockchain can also benefit document management in the pharmaceutical industry. Common applications include:
- Document traceability from its creation to every edit
- Record immutability protecting it from tampering
- Decentralized cryptographic data protection
- Global accessibility, especially when combined with cloud-based solutions
At the same time, blockchain limitations block its widespread adoption. Common issues include limited scalability and interoperability between different blockchain platforms.
Best Practices for Managing Pharmaceutical Documents
Setting up pharma regulatory document control should reflect your specific needs. Whether you consider
SAMD for management
or develop medicines, your company will benefit from these best practices:
- Document management standardization.
Document and share standards for every process, from record creation and editing to storage and archiving.
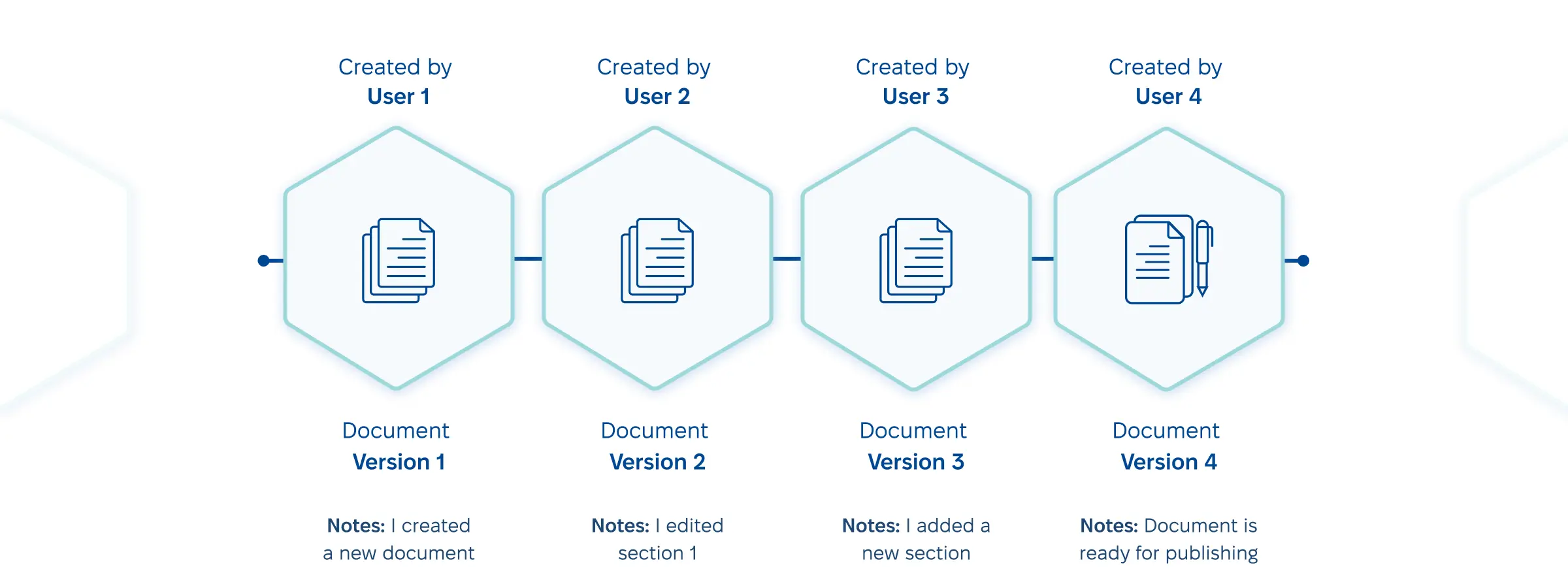
- Version control management.
To eliminate mistakes, your electronic document management system should store every past version but only provide access to the latest record.
- Records management.
The repository should list every document, its creation date, access rights, revision history, current status, etc., facilitating effective pharma document version control.
- Data security.
In addition to encryption, GxP-compliant document management should focus on role-based access and multi-factor authentication to minimize breaches and tampering.
Introducing employee training is another way to enhance the pharmaceutical quality management system. All employees should be aware of the documentation standards, policies, regulatory requirements, and potential repercussions.
Choosing the Right Document Management System
Based on Exoft’s experience in the MedTech industry and our familiarity with regulatory requirements, we have identified essential features that document management software for pharmaceuticals should possess. Our top 5 include:
- Role-based access control.
Every employee should only have access to necessary documents. This approach reduces security risks and tampering potential. But it also minimizes the number of permission requests and resulting errors.
- Advanced search tools.
Search filters must recognize file types, metadata, and files’ contents, including handwritten and scanned copies. Advanced search capabilities increase employee efficiency.
- Audit trail.
It enables system admins to follow all document changes, including editing, sharing, deletion, and downloads. Ideally, the system should include automated routines to prevent unauthorized actions and notifications about them.
- Customized reports.
An effective electronic document management system for the pharmaceutical industry should be able to generate reports to satisfy your business processes. Users should be able to adjust report data, layout, and file type.
- Scalability.
The document management system should scale up or down according to demand without consuming extra resources. Scalability is critical for companies undergoing records digitization.
You can opt for a ready-made or custom solution. The choice depends on your business scope and specific needs, as well as your implementation budget and timeline. Contact the Exoft team to schedule a free consultation to discuss your options. And see what
clients say about Exoft.
Conclusion
As regulations evolve, pharmaceutical document management solutions must address the new challenges to avoid non-compliance repercussions. Disruptive technologies, including cloud processing, AI, and blockchain, can automate documentation processes and enhance cybersecurity. Along with must-have features like access control, search filters, and audit trails, advanced technologies ensure your documentation management matches the current requirements.
If you’re considering digitization but can’t choose among ready-made solutions or want to
hire web developers
for custom development, contact Exoft. We’ll set up a consultation to address your specific business needs.
Frequently asked questions
How quickly can a document management system be implemented?
The pharma document management system can be implemented within six months. However, the specific timeline will depend on your manufacturing and operational needs, as well as the complexity of the system. The transition period should include integrating legacy systems and staff training.
Can a document management system be integrated with existing pharmaceutical software and tools?
A custom-built documentation management system can seamlessly integrate any existing tools you use. Ready-made solutions typically support integrations with the most popular tools on the market, but legacy integrations may require third-party assistance.
What features of a document management system can you offer?
Exoft offers many documentation management features, including database management, advanced search, role-based access control, MFA, audit trails, reporting, and more. With custom development, you can get any features to help you manage documentation, ensuring FDA compliance document solutions.
What types of services for effective pharmaceutical document management do you offer?
With Exoft, you get the benefits of a full range of web and mobile development services, from architecture design to quality assurance, launch, and post-launch support.