Is your lab stuck in the past?
See how we fix that in our case study.
Published: 30 April, 2025 · 8 mins read
Explore how quality management systems improve lab efficiency, traceability, and compliance in R&D, industrial, and other environments with real-world examples.
Still using spreadsheets and outdated systems to track everything in your lab? It might be time for an upgrade — one that’s totally possible with a laboratory quality management system (LQMS). Such a modern tool completely changes the way you work. It streamlines lab operations, improves traceability, and makes sure you’re always on the right side of regulations.
We’ve seen the tangible impact ourselves. When we helped our client modernize their
ESP failure analysis system, load speeds jumped by 50%, and reliability for failure diagnosis improved dramatically.
In this post, we’ll discuss what a quality management system for laboratory is, what problems it solves, and how to choose the right fit for your needs.
Key takeaways:
See how we fix that in our case study.
In any laboratory, whether you’re pushing the boundaries of scientific discovery in research or ensuring product quality in manufacturing, you have lots of processes to keep track of. A quality management system helps you do just that.
At its core, a lab QMS is a digital framework that organizes, tracks, and documents every part of your lab’s workflows, from processes and procedures to roles and responsibilities. Its main goals? To help you deliver safe, reliable products that meet industry regulations and consistently live up to customer expectations.
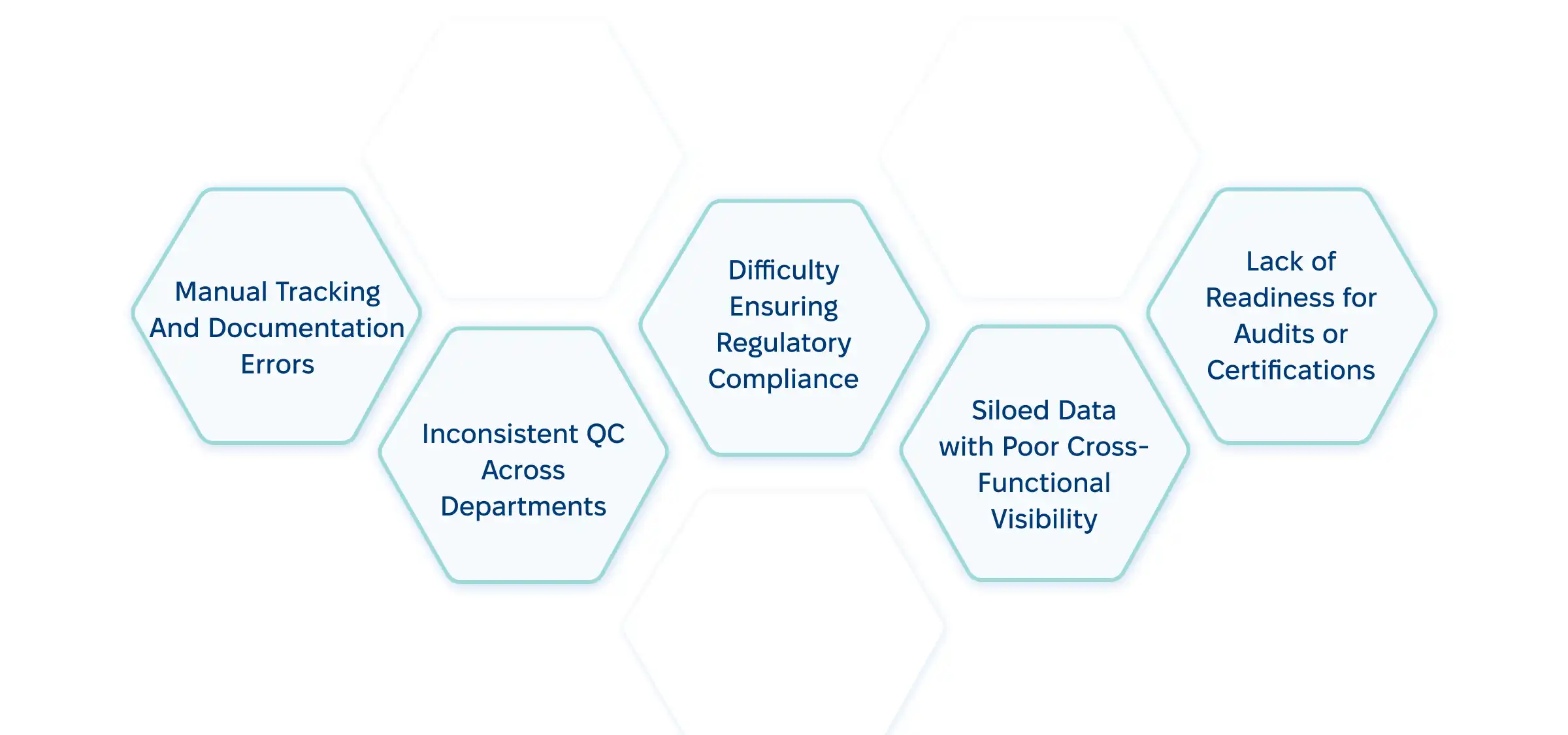
Here are some operational problems a QMS solves:
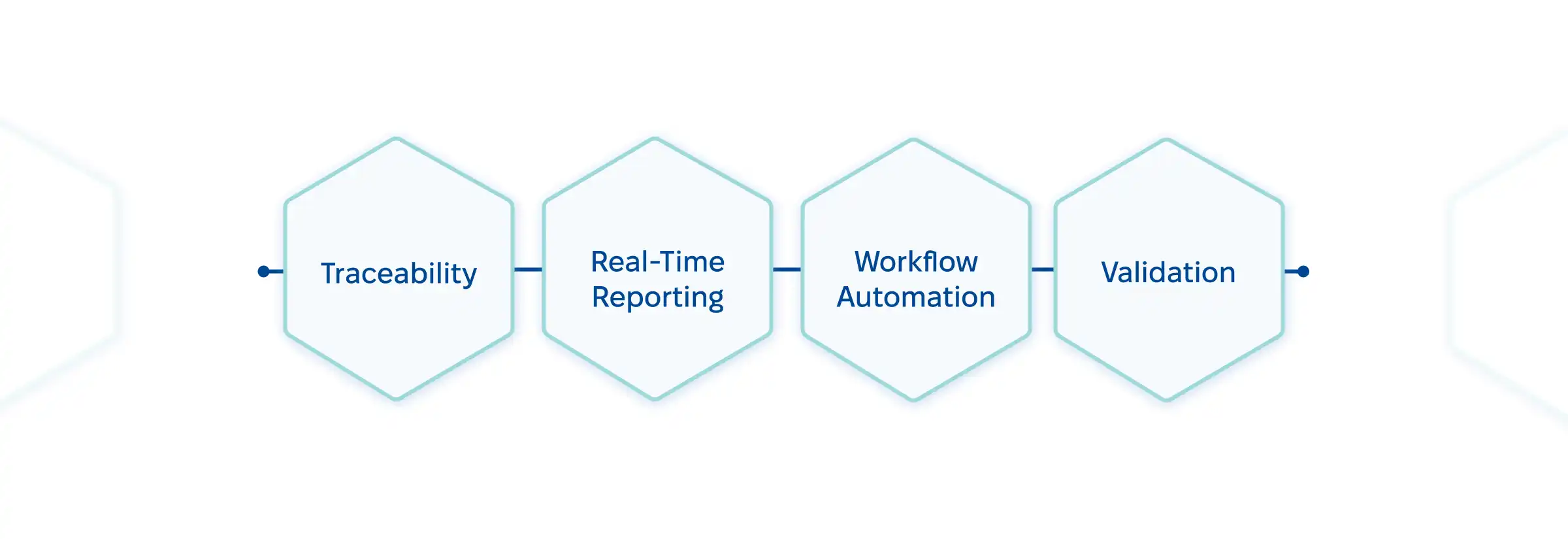
What should you actually look for in a quality management system for laboratory? Four areas have to be of your particular attention:
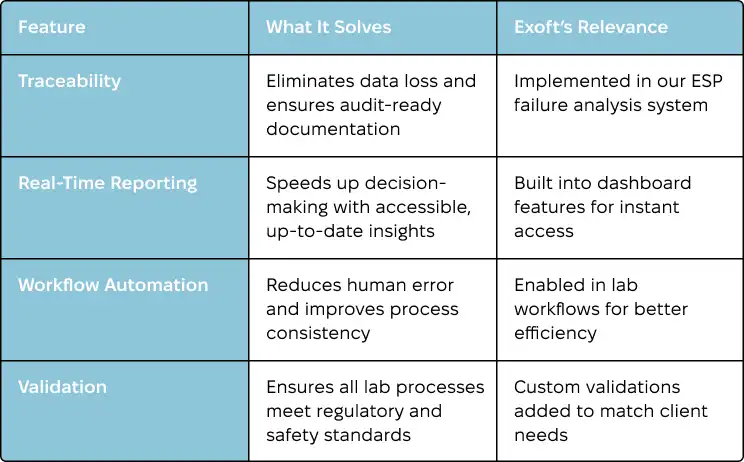
To see how these features make a difference, check out this:
| Feature | What It Solves | Exoft’s Relevance |
|---|---|---|
| Traceability | Eliminates data loss and ensures audit-ready documentation | Implemented in our ESP failure analysis system |
| Real-Time Reporting | Speeds up decision-making with accessible, up-to-date insights | Built into dashboard features for instant access |
| Workflow Automation | Reduces human error and improves process consistency | Enabled in lab workflows for better efficiency |
| Validation | Ensures all lab processes meet regulatory and safety standards | Custom validations added to match client needs |
«By adding workflow automation and traceability in our ESP modernization project, we helped our client cut manual errors by over 60%»
Picking the right lab quality management system isn’t just about checking off essential features. There are several more considerations you should go through. Let’s get them straight.
When selecting a quality management system for laboratory, you need to think about how your lab actually works right now and where you’re headed next. Several factors to pay attention to include:
If the above considerations weren’t enough to help you decide on quality management software for labs, we’ve got you covered. Here are the most frequently asked questions regarding these solutions:
Let’s look at how we brought
a legacy lab system
back to life — and made it even better.
Our client provides oil and gas companies with consulting services and software for artificial lift systems. An electrical submersible pump failure analysis app is one of their key offerings.
For years, this ESP failure analysis app served both internal teams and external operators. But over time, its performance began to lag. Slower response times, outdated tech, and limited scalability made it harder to keep up. Here’s how we solved that.
The first big challenge? Upgrading an outdated technology stack. The original app used older tools that were no longer supported. That’s why our engineers migrated LoopBack 3 to LoopBack 4, Angular 8 to Angular 14, and AWS SDK 2 to AWS SDK 3.
The next challenge — and the most painful one — was enhancing the app’s performance and usability.
To tackle that, we split the app’s codebase into smaller, more manageable units. We also improved the hierarchy and cleaned up outdated or unnecessary code. On the server side, we separated front-end and back-end instances to eliminate performance bottlenecks.
Beyond just refactoring code, we looked at the entire system, handling:
We also added a long list of highly requested features:
As a result of modernization, our client saw a 50% increase in app load speed, smoother performance, and greater reliability. Thanks to the improved architecture and feature set, monitoring ESP failures is now faster, easier, and far more insightful.
Explore our post on
As we said, lots of laboratories still rely on manual tracking and disconnected systems. That’s the primary reason to switch to custom lab management solutions. Here’s what these systems bring to the table:
If you’d like to reap the benefits we’ve mentioned with modern laboratory business apps, count on Exoft. We’ve been building, supporting, and scaling lab systems for over 10 years. Whether you’re looking to augment your IT team, create a custom LIMS or LQMS, or simply hire a dedicated development team — we’ve got the expertise to make it happen.
Adopting a quality management system for laboratory is an absolute must-have. Why? It helps you bring clarity, consistency, and control to your processes. And consider this: you reduce manual errors, improve traceability, speed up reporting, and stay audit-ready along the way.
At Exoft, we’ve spent years building top healthcare mobile apps with lab process automation tools included. Therefore, we know how much these solutions support high-stakes, high-precision environments. We also know what it takes to design tools that truly make labs smarter.
Let’s discuss your project!
Excel and paper logs might be great for basic tasks, but they’re not built for quality management in the laboratory. Digital lab systems automate workflows, track every change, and centralize data. Unlike spreadsheets, they support validation, user role management, and even connect with other digital lab instruments — features you won’t get from a manual system.
Without a modern industrial lab software strategy, you face several risks. The most critical ones include data loss, inconsistent quality, and failed audits. All in all, it gets harder to trace what went wrong (or prove what went right) when information is siloed or missing.
It all depends on your lab’s size, needs, and existing infrastructure. A basic solution could take a few months, while a more complex, fully custom platform might take 6 months or longer. Need to know a precise timeline? Reach out to Exoft. We’ll start with an assessment phase, defining the right scope before building.
Of course. We’ve built digital systems for everything from ESP failure diagnostics to custom LIMS. So, regardless of your industry, you can adapt QMS tools to your specific workflows, data models, and compliance needs.
Our company skillfully combines a 10-year background in custom software development with expertise in lab-focused solutions. We can either build software from scratch or modernize existing tools. If you need a partner who gets both tech and lab operations, you’ve found the right company.